Biosimilar
Description
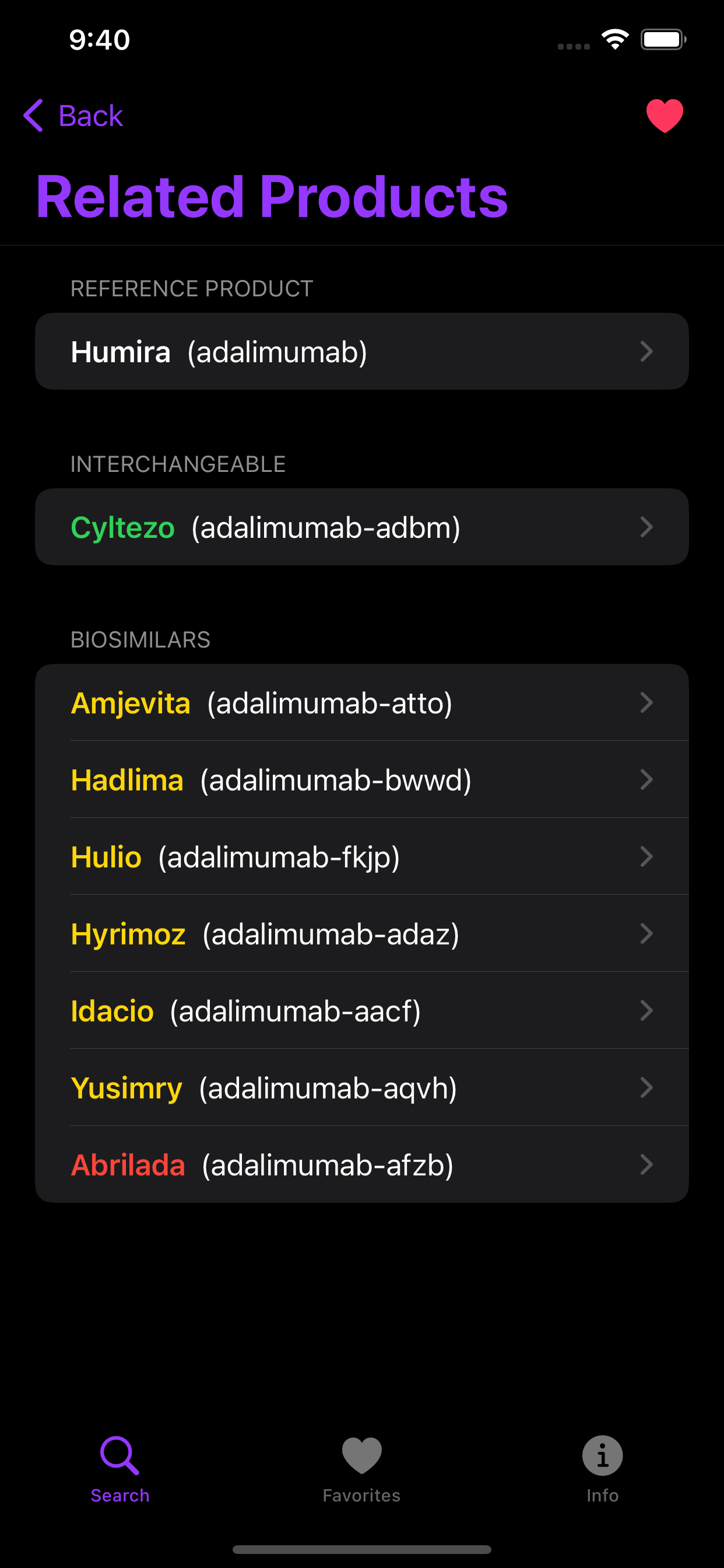
Biologic drugs offer important treatment options for patients suffering from auto-immune disorders such as rheumatoid arthritis and for common cancers including breast and colon. Biosimilar and interchangeable products, which can loosely be thought of as ‘generic’ biologics, are important for promoting competition and driving down costs. The FDA maintains a list of approved biosimilars in the Purple Book. The Biosimilar iOS app provides a handy reference to the Purple Book as well as important information related to prescribing and dispensing for clinicians and pharmacists navigating this complex area of drug therapeutics.
Features
Stoplight coloring for quick assessment of biosimilars and interchangeables
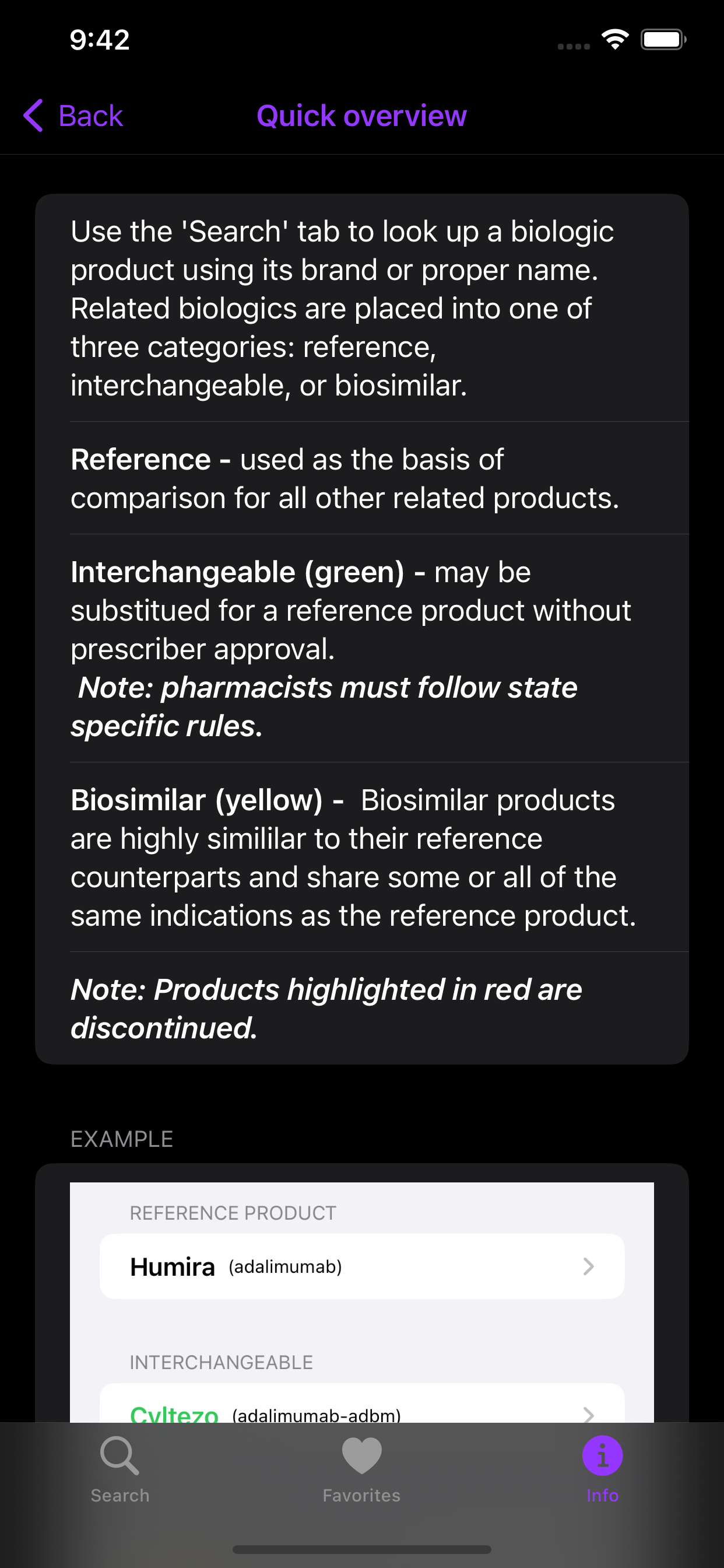
Educational references for the biosimilar approval process, naming conventions used in biologics, and more
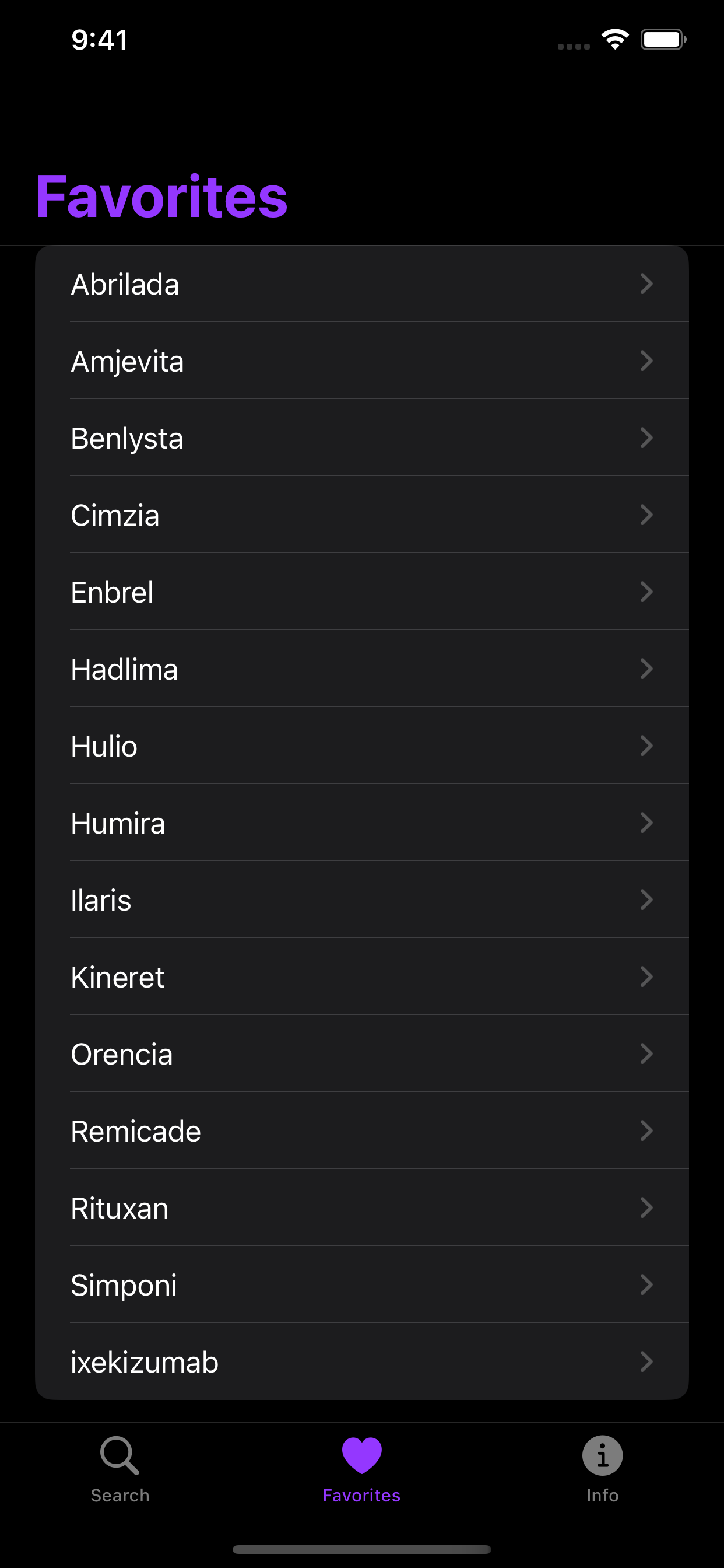
Store your most frequently prescribed/dispensed products as favorites
Requirements
iOS 16.0 or higher
Wi-Fi/internet connectivity